Sanofi and Regeneron are putting their hopes in IL6 inhibitor Kevzara as a possible therapy for COVID19 with clinical trials underway Now, a second IL6COVID19 Vaccine and Immunisation Programme (the Programme) run by the Ministry of Health (the Ministry) This is New Zealand's largest ever immunisation IL 12/23 inhibitors, IL 23 inhibitors Note this list is not exhaustive but provides a guide on the types of scenarios where a consumer should receive a third primary doseCOVID19 infection on IL23 inhibition COVID19 infection on IL23 inhibition Dermatol Ther Nov;33(6)e133 doi /dth133 Epub Jul 14 Authors Catherine J Wang 1 Interleukin23 / antagonists & inhibitors* Male Psoriasis / drug therapy*

All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt
Il 12/23 inhibitor covid
Il 12/23 inhibitor covid-TNF receptors, interleukin (IL)6 receptor inhibitors, IL17 inhibitors, IL 12/23 inhibitors, IL23 inhibitors • to advise on the appropriate timing of a third primary dose of COVID19 vaccine taking in to consideration the halflife of the medicine and expected nadir of immunosuppression as advised by JCVI, where this IL12/23 (JAK2/TYK2) IFNa/b (JAK1/TYK2) The Multiarm trial of Inflammatory Signal Inhibitors for COVID19 (MATIS) study is a twostage, openlabel, randomised controlled trial assessing the efficacy of ruxolitinib (RUX) and fostamatinib (FOS) individually, compared to standard of care in the treatment of COVID19 pneumonia N = 171 in




Autoimmune Diseases And Immune Checkpoint Inhibitors For Cancer Therapy Review Of The Literature And Personalized Risk Based Prevention Strategy Annals Of Oncology
Using a newly developed COVID19 Inflammation Score (CIS), patients were prospectively stratified for targeted inhibition of cytokine signalling by the Janus Kinase 1/2 inhibitor ruxolitinib (Rux) Ustekinumab inhibits the p40 subunit on the IL12 and 23 cytokines, and was shown in two phase III trials to be effective for both skin and joint manifestations of PsA It In the IL23 inhibitor group, filled circles represent participants receiving IL23p19 inhibitors and hollow circles represent participants receiving an IL12/23p40 inhibitor Spearman correlation between Tcell responses (cytokinesecreting cells per 10 6 PBMCs) and humoral immune responses as determined by ELISA and neutralisation assays
IL1 inhibitors, IL6 inhibitors, JAK inhibitors and belimumabtreated patients showed the lowest incidence of COVID19 among adult patients with rheumatic diseases We found no differences in sex or rheumatological disease between patients who tested positive for COVID19 and patients who tested negative Baricitinib is a JAK inhibitor which blocks multiple cytokine pathways (eg, IL2, IL6, and IL12), while potentially having fewer systemic side effects than steroid The ACTT2 trial demonstrated that baricitinib reduced the risk of progressing to intubation (in a population of patients who were not receiving steroid) First to market was Johnson & Johnson's dual IL12/IL23 inhibitor Stelara (ustekinumab) for psoriasis, which rapidly achieved blockbuster status with addon indications in psoriatic arthritis and Crohn's and made $4bn in sales last year Stelara has since been joined by J&J's followup IL23 inhibitor Tremfya (guselkumab), Sun Pharma's
PF biochemical and antiviral activity ( A) PF is a reversible inhibitor of SARSCoV2 M pro as demonstrated by recovery of enzymatic activity following a 100fold dilution of the enzyme inhibitor complex Compound 7 (PF), an irreversible inhibitor, was included as a control Treatment with antiIL6, antiIL6R antagonists or Janus kinase inhibitor (JAKi) within 48 hours of first dose of study treatment No other investigational therapies with the intent to treat the patient's COVID19 can be administered while the patient is enrolled in the studyYou're able to receive an additional dose of mRNA COVID19 vaccine if you're receiving CART therapy, received a stem cell transplant within 2 years or received a stem cell transplant and taking immunosuppressive medication IL 12/23 inhibitors Ustekinumab (Stelara) IL 23 inhibitors Guselkumab (Tremfya, Tremfya OnePress), Risankizumab




Risk Of Severe Covid 19 Outcomes Associated With Immune Mediated Inflammatory Diseases And Immune Modifying Therapies A Nationwide Cohort Study In The Opensafely Platform Medrxiv




Cutting Edge Il 23 Cross Regulates Il 12 Production In T Cell Dependent Experimental Colitis The Journal Of Immunology
FDA safety warnings on a pair of JAK inhibitor rivals helped, but with competition mounting, J&J had to slash the price of its IL12/23 inhibitor to stay competitive The IL23 inhibitors guselkumab, tildrakizumab, and risankizumab selectively target the p19 subunit and inhibit IL23, but not IL12 IL12 is now thought to have antiinflammatory properties in psoriasis, playing a role in defence against intracellular pathogens, and inhibiting IL23 alone has been shown to be preferable to inhibiting IL12/ILIL6mediated CRS in severe COVID 19, and highlighted the rationale for the use of antiIL 6 agents and key information regarding the potential features of these IL6 inhibitors in COVID19 patients Key words COVID19, cytokine release syndrome, interleukin6, interleukin6 inhibitor Introduction On 11 March , the World Health




Navigating Immunosuppression In A Pandemic A Guide For The Dermatologist From The Covid Task Force Of The Medical Dermatology Society And Society Of Dermatology Hospitalists Journal Of The American Academy Of




Covid 19 And Immunomodulator Immunosuppressant Use In Dermatology Journal Of The American Academy Of Dermatology
In our study, no patients treated with IL17A or IL12/23 inhibitors have been confirmed for SARSCoV2 infection Overall, 16 patients out of the 17 analysed (941%) fully recovered, regardless of the type of RMD and the type of immunomodulation considered To evaluate whether IL17, IL12/23 or TNF inhibitors are associated with an increased risk for serious infection in realworld patients with psoriasis or PsA, Li In our study, no patients treated with IL17A or IL12/23 inhibitors have been confirmed for SARSCoV2 infection Overall, 16 patients out of the 17 analysed (941%) fully recovered, regardless of the type of RMD and the type of immunomodulation considered




Risk Of Severe Covid 19 Outcomes Associated With Immune Mediated Inflammatory Diseases And Immune Modifying Therapies A Nationwide Cohort Study In The Opensafely Platform Medrxiv




The Other Side Of The Moon A Clinical Dialogue On The Il 23 Pathway European Medical Journal
IL12/23 or IL23 inhibitors include such therapies as Stelara, Tremfya, and Skyrizi;Apilimod Apilimod ( STA5326) is a drug that was initially identified as an inhibitor of production of the interleukins IL12 and IL23, and developed for the oral treatment of autoimmune conditions such as Crohn's disease and rheumatoid arthritis, though clinical trial results were disappointing and development for these applications was not have been cited in media articles as potential treatment options for COVID1911, 12 Twelve other IL6 inhibitors are in clinical or preclinical development13 No published clinical trials were identified assessing the efficacy and safety of IL6 inhibitors in managing COVID19




Morbus Crohn Entzundung Stoppen Remission Erhalten Pz Pharmazeutische Zeitung




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt
We recommend the initiation and continuation of lowrisk immunomodulating drugs, such as Interleukin (IL)17, IL12/23, and IL23 inhibitors, for treatment of PSO during COVID19 era For psoriatic patients with comorbidities switching to safer modalities such as systemic retinoids, apremilast, and home phototherapy is recommended Furthermore, last month, Roche's IL6 inhibitor Actemra/RoActemra (tocilizumab) also failed to show a benefit in an Italian study involving patients withSARSCoV2 Infections Among Vaccinated Individuals with Rheumatic Disease Results from the COVID19 Global Rheumatology Alliance Provider Registry belimumab, CD_ inhibitors, IL_1 inhibitors, IL6 inhibitors, IL_12/23 inhibitors, IL_17 inhibitors, anti




The Role Of Interleukin 17 In Asthma A Protective Response European Respiratory Society




Targeting Interleukin 23 In The Treatment Of Noninfectious Uveitis Ophthalmology
Results The study population (n=) included patients with psoriasis receiving methotrexate (n=14), TNF inhibitors (n=19), IL17 inhibitors (n=14), IL23 inhibitors (n=) and healthy controls (n=15), who had received both vaccine doses The median age of the study population was 44 years (IQR 33–52), with 43 (52%) males and 71 (87% Tocilizumab, sarilumab and siltuximab, which are commercially available IL6 inhibitors, have been cited in media articles as potential treatment options for COVID1912, 13 Twelve other IL6 inhibitors are in clinical or preclinical development14 Published evidence for the use of IL6 inhibitors to treat COVID19 is limited IL17 and IL17 receptor inhibitors boast an impressive safety history and are widely available Here, we argue that targeting IL17 is
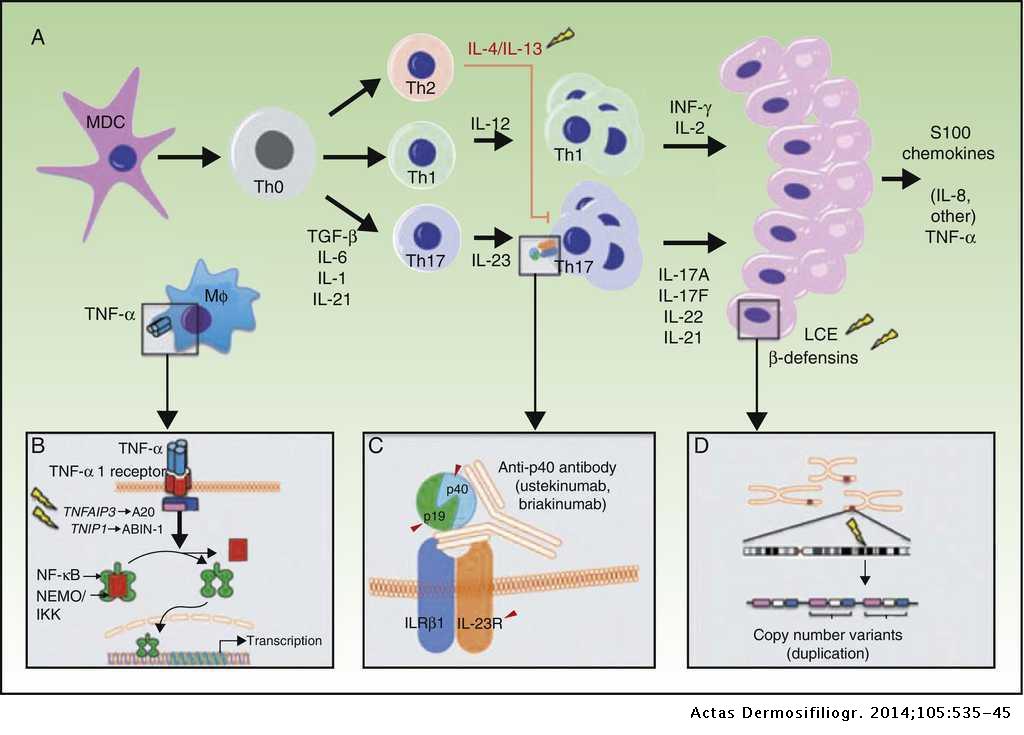



The Pathogenesis And Genetics Of Psoriasis Actas Dermo Sifiliograficas




Next Generation Of Biologics For The Treatment Of Crohn S Disease An Ceg
Background There is uncertainty about the associations of angiotensive enzyme (ACE) inhibitor and angiotensin receptor blocker (ARB) drugs with COVID19 disease We studied whether patients prescribed these drugs had altered risks of contracting severe COVID19 disease and receiving associated intensive care unit (ICU) admission Methods This was a prospective cohort studyThe other groups ('AntiTNF agents', 'IL12/23 inhibitors', 'Antiintegrin agents' and 'JAK inhibitor') were compared to those did not receive the corresponding biologics FIGURE 3 Figure 3 The association of biologics and ICU admission rate among IBD patients with COVID19 according to data from SECUREIBD ( 27 ) IL23 inhibitors target a type of cytokine called IL23 Cytokines are a class of proteins that help transmit signals from one cell to another




Critical Determinants Of Cytokine Storm And Type I Interferon Response In Covid 19 Pathogenesis Clinical Microbiology Reviews




Psoriasis Translating Current And Emerging Therapies Into Enhanced Management Strategies Youtube
About a year and a half after presenting positive topline results in Crohn's disease, Eli Lilly gave its experimental IL23 antiinflammatory mirikizumab a boost Realworld data also suggest that IL12/23 inhibitors may be used safely during the COVID19 pandemic5 In the New York–based case series examining patients with IMID, ustekinumab was not associated with increased rates of COVID19Interim protocol Genomic surveillance of patients with COVID19 who are treated with neutralising monoclonal antibody or immunosuppressed 5 inhibitors, IL17 inhibitors, IL 12/23 inhibitors, IL 23 inhibitors (Note this list is not exhaustive) Groups A and B2 The following patients may be entered in surveillance at




Acr Patients With Rheumatic Disease Should Receive Covid 19 Vaccine As Soon As Possible
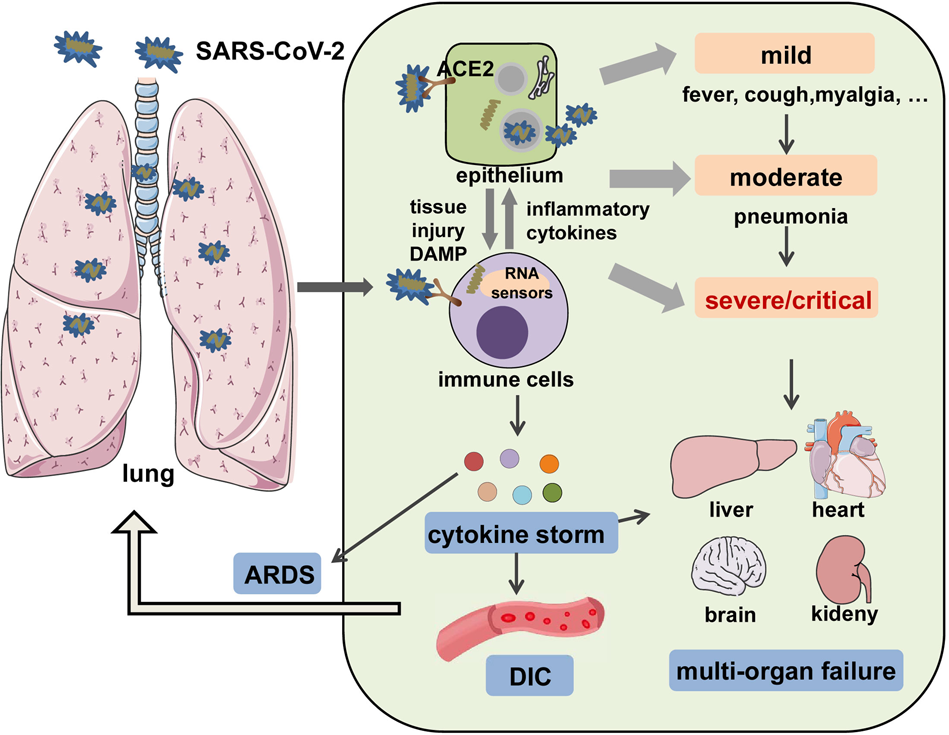



The Signal Pathways And Treatment Of Cytokine Storm In Covid 19 Signal Transduction And Targeted Therapy
Researchers say they have cut deaths by a quarter in patients who are sickest with COVID19 Just like Yadegar has found, IL6 inhibitors can lead toWe interpret that to mean at least 4 weeks of medications (to cover quarantine period plus a 2week buffer) Many patients request up to a 12week supply of key medicines, which may be appropriate if safe, safely stored and medically appropriate More advice on home preparedness for COVID19 can be found at the Centers for Disease Control (CDC)Soluble TNF receptors, interleukin (IL)6 receptor inhibitors, IL17 inhibitors, IL 12/23 inhibitors, IL23 inhibitors • to advise on the appropriate timing of a third primary dose of COVID19 vaccine taking in to consideration the halflife of the medicine and expected nadir of




Covid 19 In Immune Mediated Inflammatory Diseases Case Series From New York Nejm




An Evidence Based Guide To Sars Cov 2 Vaccination Of Patients On Immunotherapies In Dermatology Sciencedirect
IRAK 4 Inhibitor (PF) in Hospitalized Patients With COVID19 Pneumonia and Exuberant Inflammation 9 and 10, and the interleukin (IL)1 family receptors (IL1R, IL18R and IL33R) that mediate much of the innate immune signaling Other medical condition other than COVID19 or laboratory abnormality that may increase the risk of IL1 inhibition, but not IL6 inhibition, was associated with a significant reduction of mortality in patients admitted to hospital with COVID19, respiratory insufficiency, and hyperinflammation IL6 inhibition was effective in a subgroup of patients with markedly high Creactive protein concentrations, whereas both IL1 and IL6 inhibition were effective in patients Targeted therapies for chronic inflammatory diseases, including TNFinhibitors, IL12 and IL23 inhibitors, and integrin inhibitors, only had a




Il 12 Regulates Type 3 Immunity Through Interfollicular Keratinocytes In Psoriasiform Inflammation




The Society For Immunotherapy Of Cancer Perspective On Regulation Of Interleukin 6 Signaling In Covid 19 Related Systemic Inflammatory Response Journal For Immunotherapy Of Cancer
During severe COVID19, the heightened levels of IL1β in several patients with COVID19 (11, 12) indicate the formation of an NLRP3 inflammasome that converts pro–IL1β to mature IL1β BTK binds to and phosphorylates NLRP3, thereby promoting its oligomerization and assembly into an inflammasome (24–26) With more than 81 000 deaths worldwide from coronavirus disease 19 (COVID19) by ,1 it is incumbent on researchers to accelerate clinical trials of any readily available and potentially acceptably safe therapies that could reduce the rising death toll Severe acute respiratory syndrome coronavirus 2 (SARSCoV2) gains access to host cells via Sarilumab is an IL6 receptor antagonist that was approved for use in rheumatoid arthritis in 17 The rationale for using this agent for COVID19 coronavirus infection is




Should We Stimulate Or Suppress Immune Responses In Covid 19 Cytokine And Anti Cytokine Interventions Sciencedirect




Covid 19 Vaccines For Rheumatic Diseases Guidance From The American College Of Rheumatology
Since the novel coronavirus is a type of viral respiratory infection, the results also encourage further investigation into a potential relationship between ILIntegrin inhibitors include Entyvio The other good news was that combining therapies, such as taking methotrexate along with a TNF inhibitor, did not Biologics like IL17 and IL12/23 inhibitors are also used to treat the condition Biologics like TNF inhibitors, however, may increase the risk of infections and lower the body's ability to fight infections, including COVID19



2



Coronavirus Covid 19 Immunocompromised Government Of Nova Scotia Canada
Contagion, August , Volume 5, Issue 4 Use of an IL6 inhibitor has the potential to prevent the cytokine storm caused by severe COVID19 infection Interleukin6 (IL6) is a cytokine responsible for organ development, inflammation, and immune responses 1 As a key stimulator of acute phase proteins, IL6 is associated with proinflammatory




Redox Imbalance Links Covid 19 And Myalgic Encephalomyelitis Chronic Fatigue Syndrome Pnas




Jcm Free Full Text Biologics For Psoriasis During The Covid 19 Pandemic Html
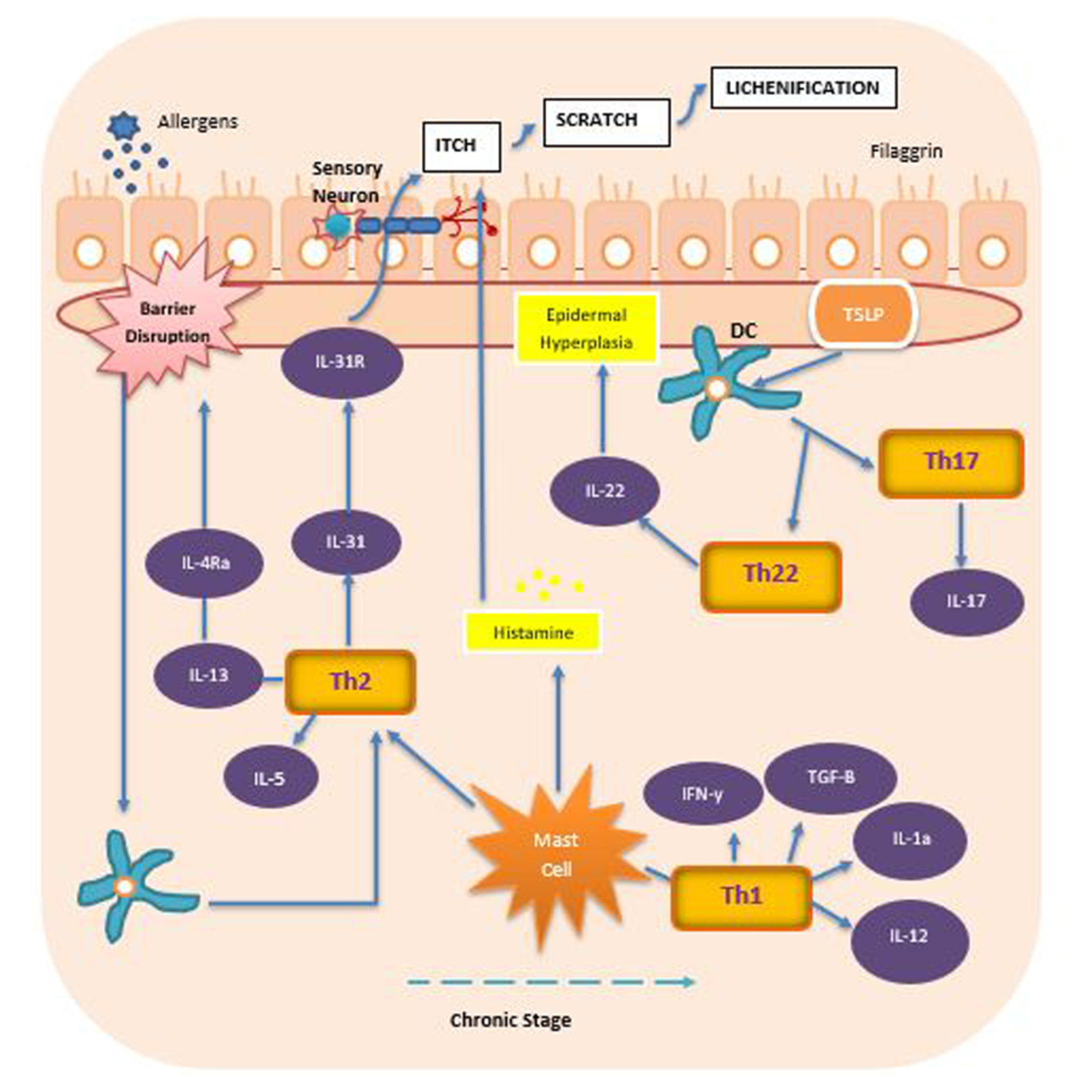



Cureus Review Of Prominent Cytokines As Superior Therapeutic Targets For Moderate To Severe Atopic Dermatitis




Clinical Features And Prognostic Factors In Covid 19 A Prospective Cohort Study Ebiomedicine




Therapeutic Agents Rounding The Immunopathology Of Covid 19 Tcrm
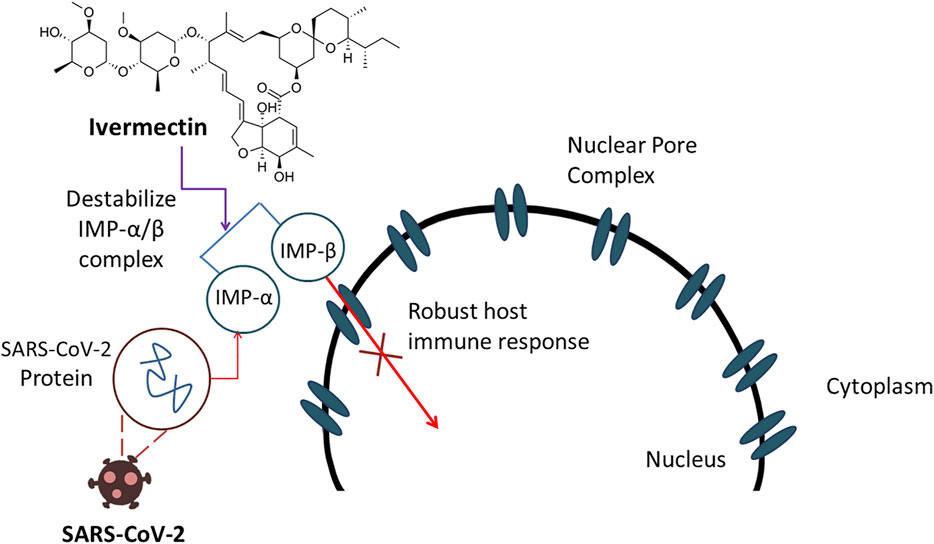



Frontiers Therapeutic Effectiveness And Safety Of Repurposing Drugs For The Treatment Of Covid 19 Position Standing In 21 Pharmacology




H Covid 19 And Skin Diseases
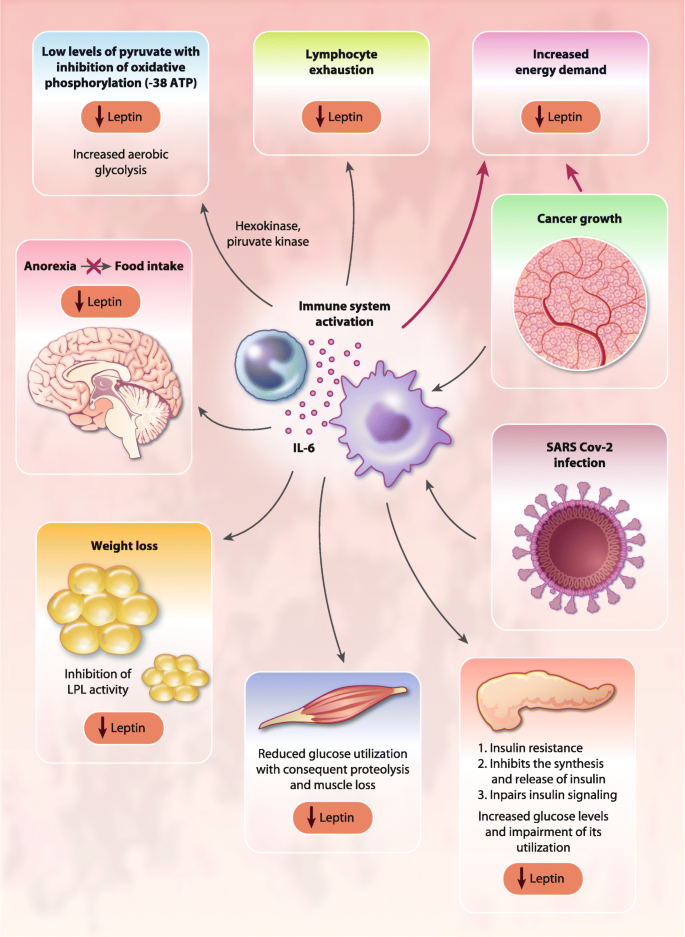



Covid 19 And Cytokine Storm Syndrome Can What We Know About Interleukin 6 In Ovarian Cancer Be Applied Journal Of Ovarian Research Full Text
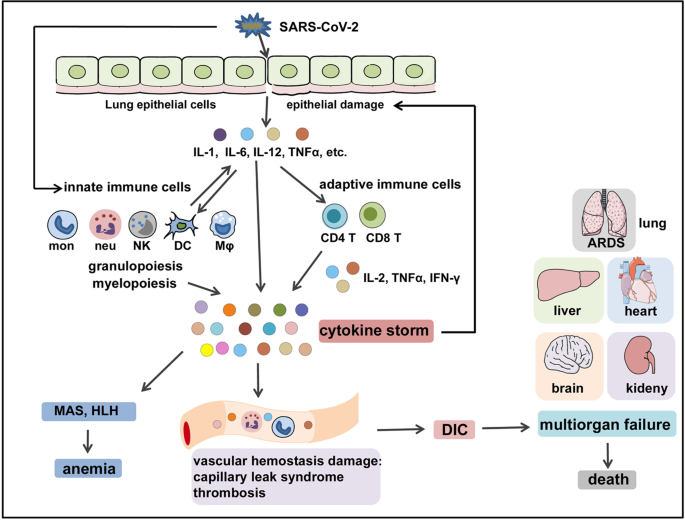



The Signal Pathways And Treatment Of Cytokine Storm In Covid 19 Signal Transduction And Targeted Therapy




Outcomes Of Patients With Chronic Plaque Psoriasis And Hidradenitis Suppurativa On Biologic Therapy During The Covid 19 Pandemic A Uk Dermatology Tertiary Centre Experience Khan 21 Skin Health And Disease Wiley Online Library




Imperfect Storm Is Interleukin 33 The Achilles Heel Of Covid 19 The Lancet Rheumatology
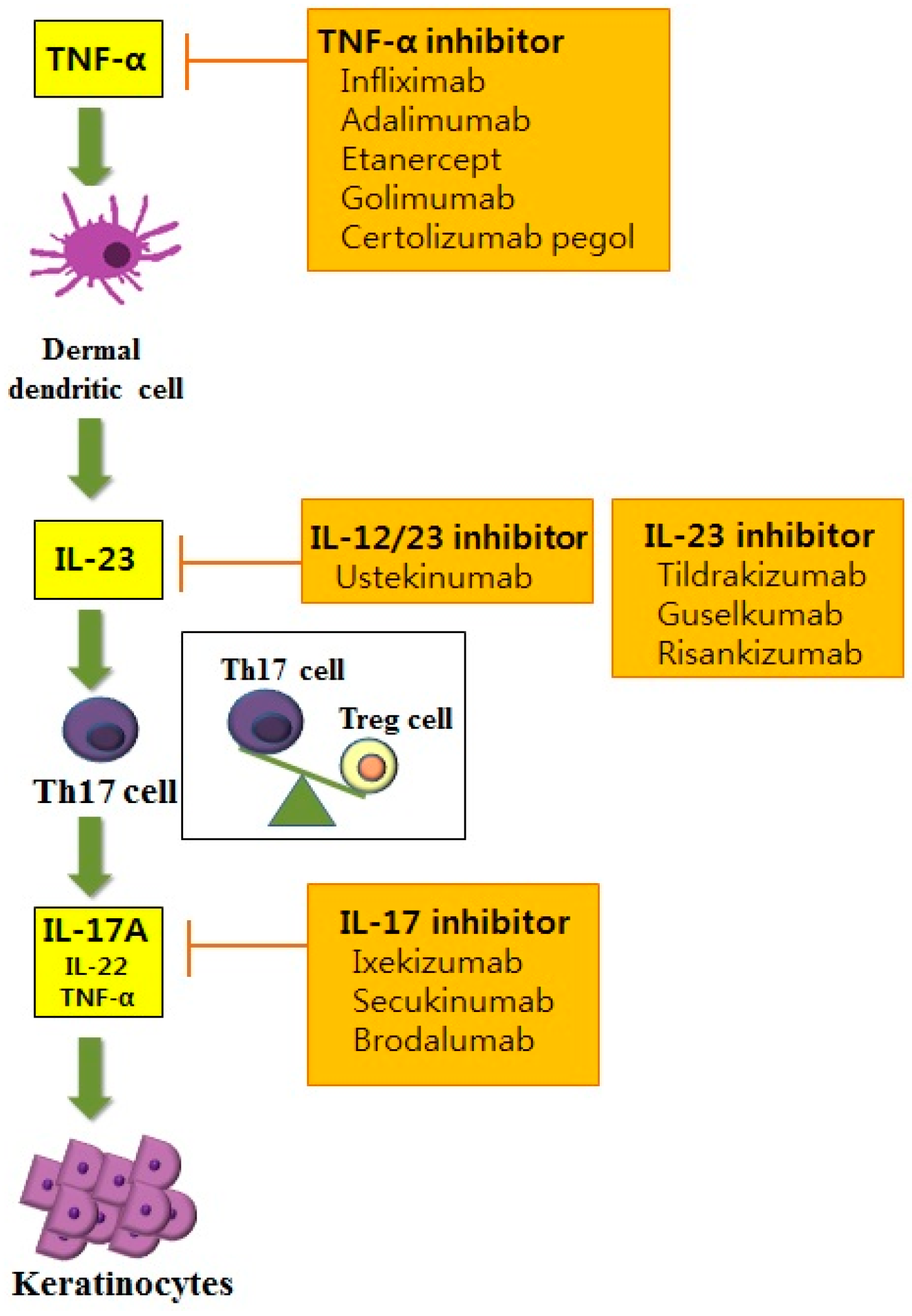



Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html




Interleukin 17 A Potential Therapeutic Target In Covid 19 Journal Of Infection




Gisg Corona Ced Newsletter 5 Vom 10 Gastroenterologische Facharztpraxis Am Mexikoplatz




Advancing The Treatment Of Psoriatic Arthritis Focus On The Il 23 Pathway European Medical Journal



2
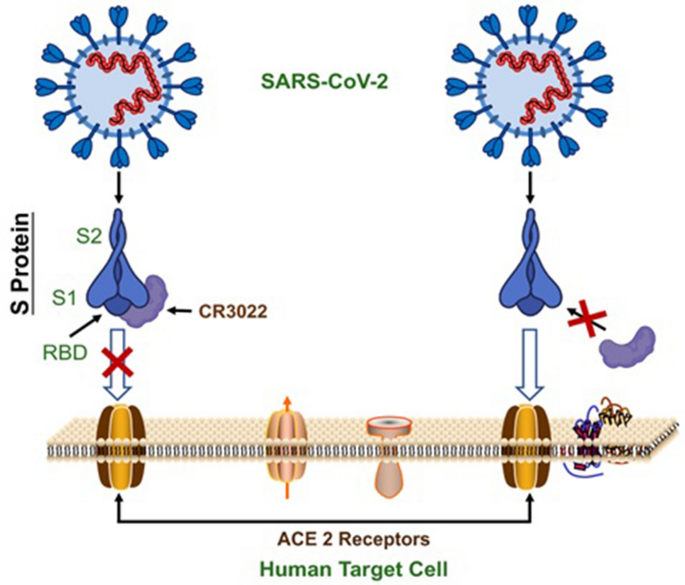



Current Strategies Against Covid 19 Chinese Medicine Full Text




A Case For Targeting Th17 Cells And Il 17a In Sars Cov 2 Infections The Journal Of Immunology



2




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology



Psoriasis Il 23 Inhibitor Guselkumab Ist Neue Option Fur Schwer Behandelbare Korperstellen




Covid 19 And Immunomodulation In Ibd Gut




Circulating Markers Of Neutrophil Extracellular Traps Are Of Prognostic Value In Patients With Covid 19 Arteriosclerosis Thrombosis And Vascular Biology
:max_bytes(150000):strip_icc()/Ankylosing-spondylitis-covid-19-5104733_Final-d8a8daecde594203949d5748d67c4dd4.jpg)



Managing Ankylosing Spondylitis During The Covid 19 Pandemic
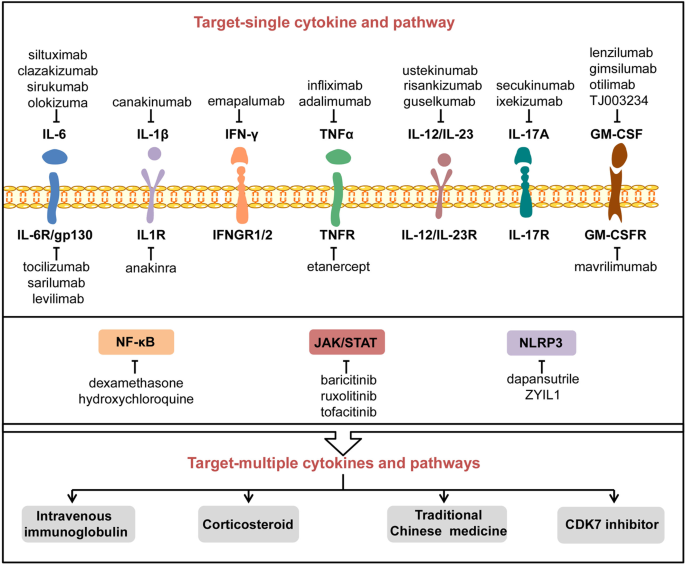



The Signal Pathways And Treatment Of Cytokine Storm In Covid 19 Signal Transduction And Targeted Therapy




Inhibition Of Pikfyve Kinase Prevents Infection By Zaire Ebolavirus And Sars Cov 2 Pnas




A Human Interleukin 12 23 Monoclonal Antibody For The Treatment Of Psoriasis Nejm




d Wichtige News Zur Behandlung Der Psoriasis Rosenfluh Ch
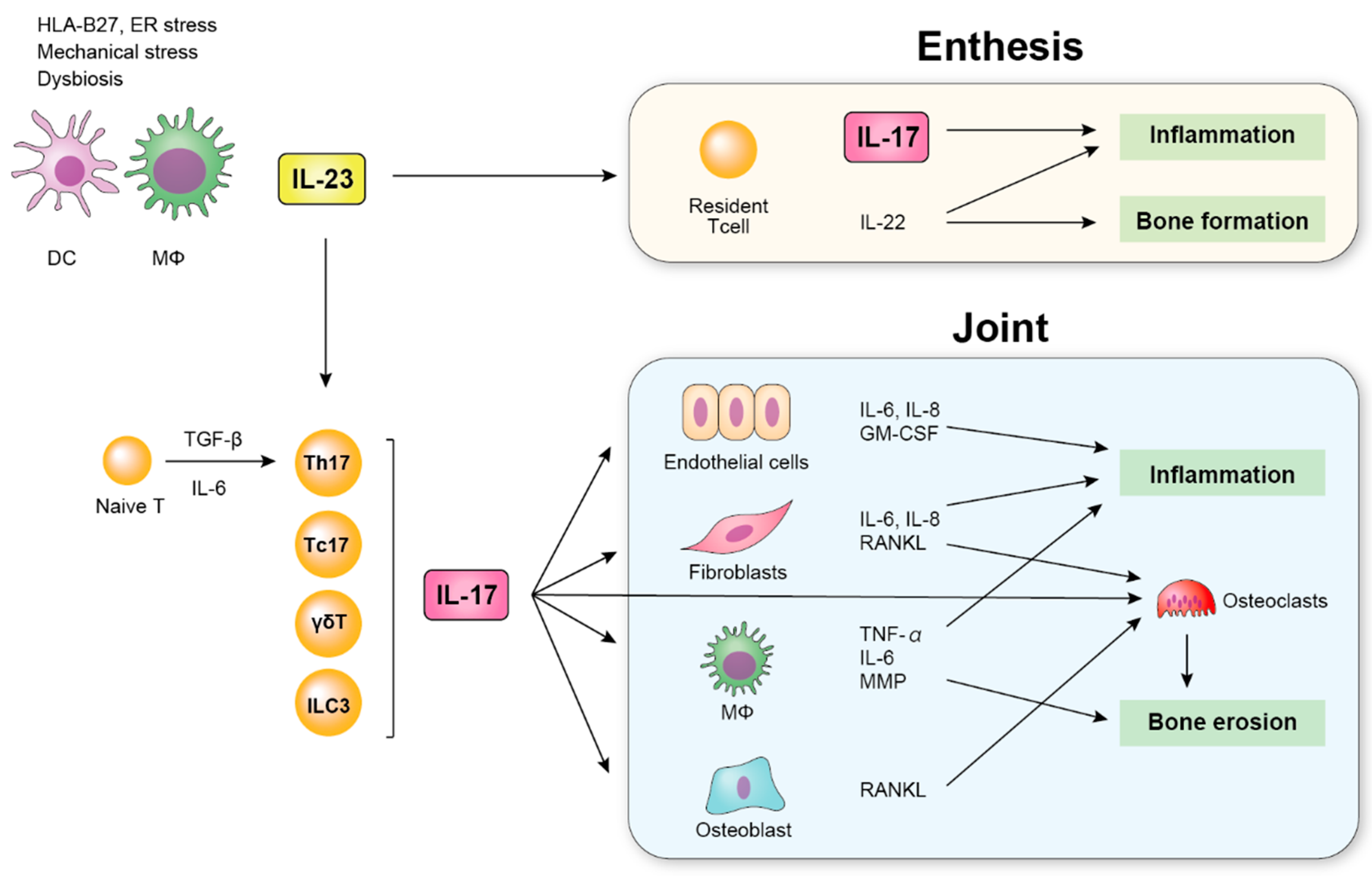



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html




Patients With Ibd Have Robust Response To Covid 19 Vaccines



Pdf Covid 19 Infection On Il 23 Inhibition




The Interplay Between Inflammatory Pathways And Covid 19 A Critical Review On Pathogenesis And Therapeutic Options Sciencedirect
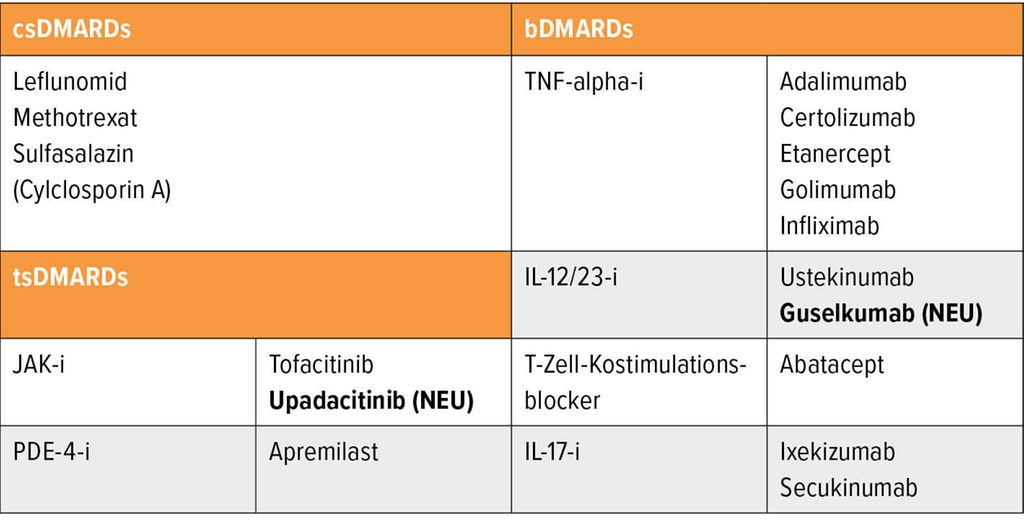



Neue Therapien Und Impfansprechen Bei Autoimmunerkrankungen Rheumatologie Universimed Medizin Im Fokus




Autoimmune Diseases And Immune Checkpoint Inhibitors For Cancer Therapy Review Of The Literature And Personalized Risk Based Prevention Strategy Annals Of Oncology




T Helper 17 Response To Severe Acute Respiratory Syndrome Coronavirus 2 A Type Of Immune Response With Possible Therapeutic Implications Viral Immunology
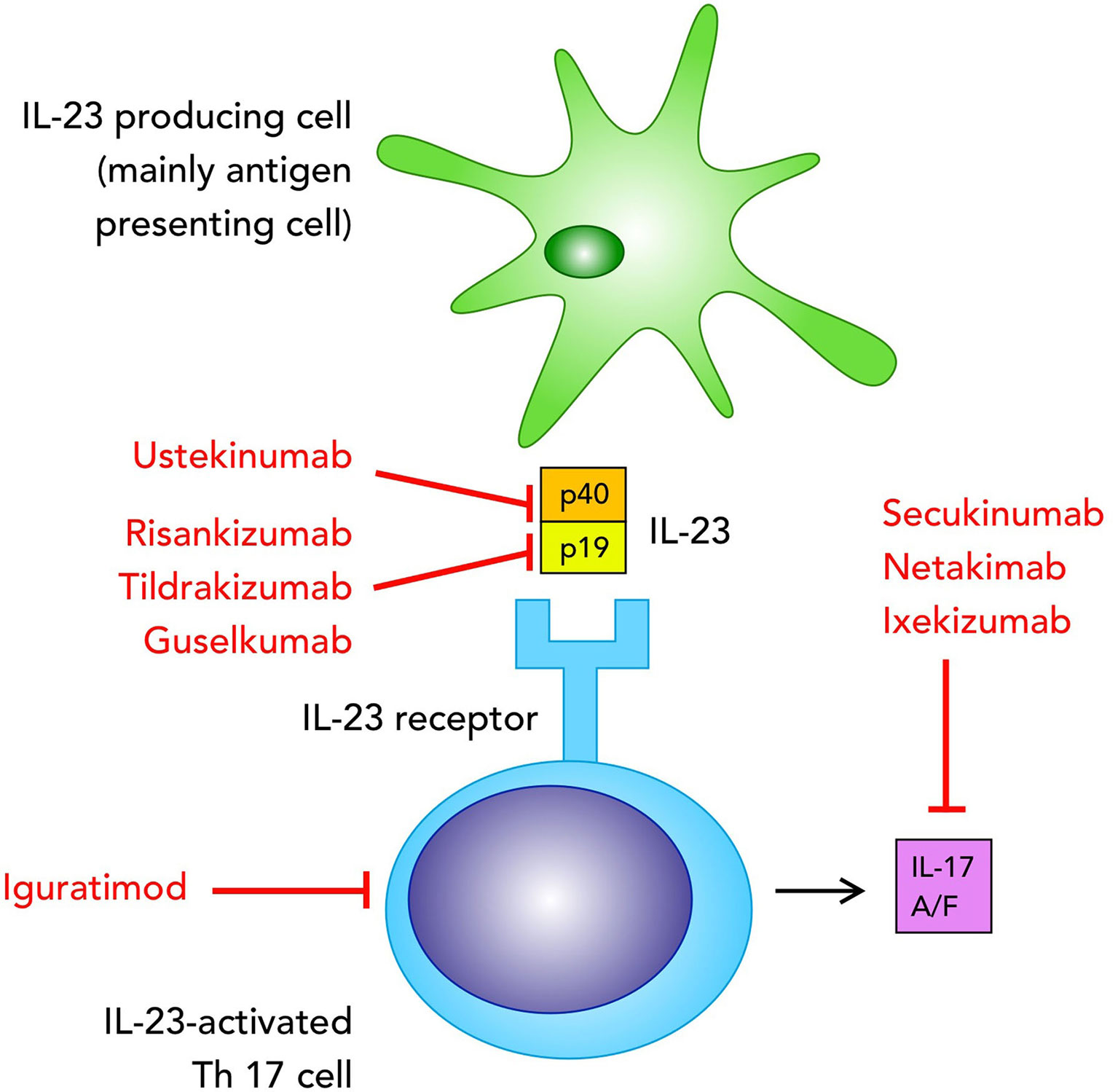



Frontiers Clinical Trials Supporting The Role Of The Il 17 Il 23 Axis In Axial Spondyloarthritis Immunology




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt



Human Immune Response To Sars Cov 2 What Is Known A Scoping Review
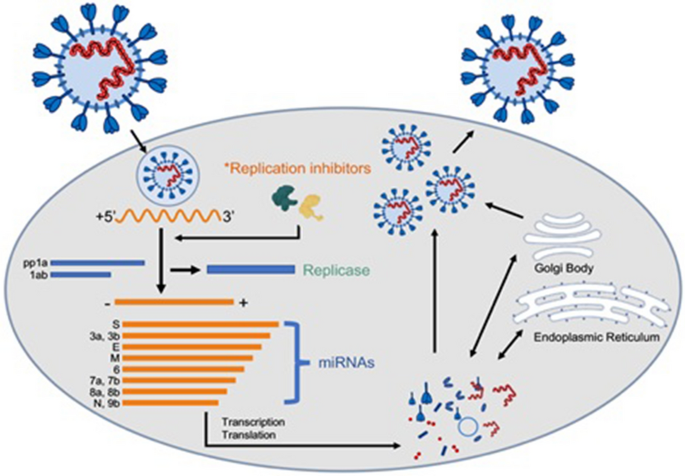



Current Strategies Against Covid 19 Chinese Medicine Full Text
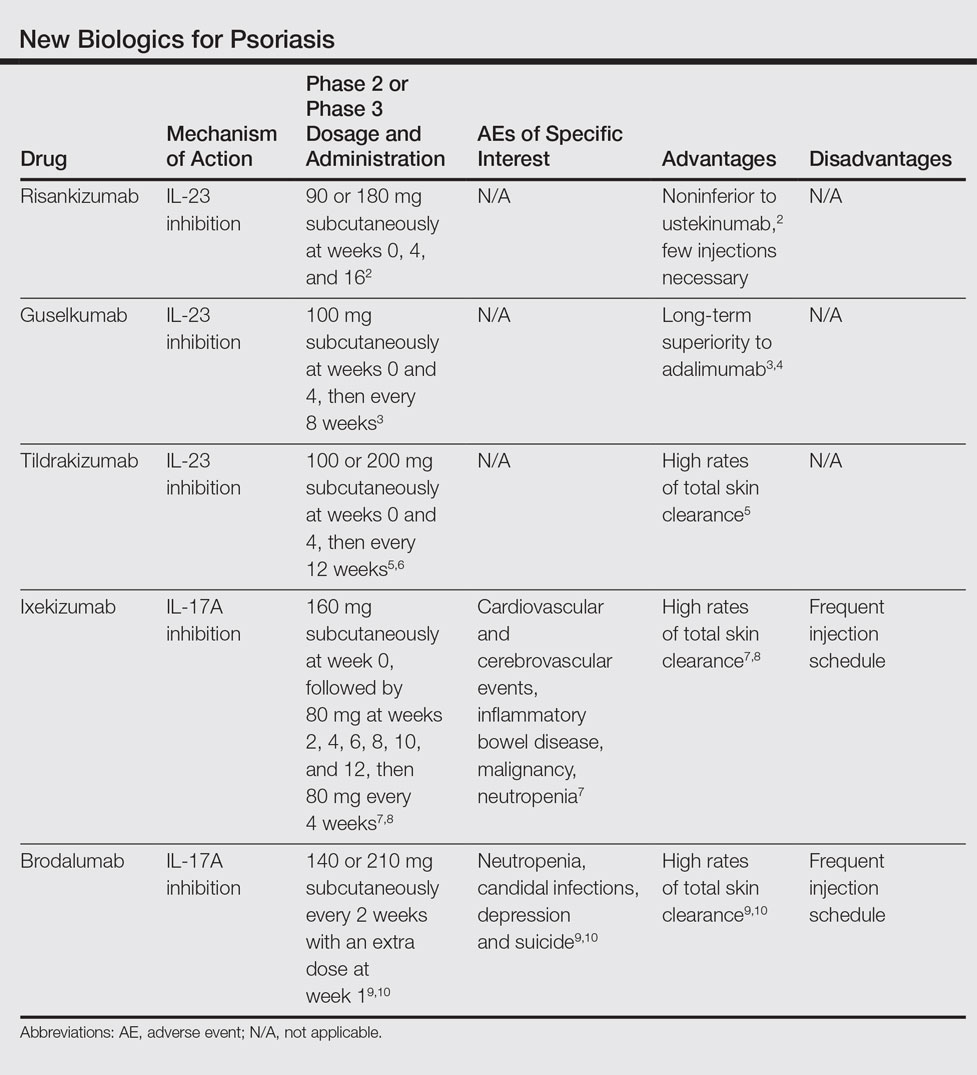



New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology




Novel Coronavirus Sars Cov 2 Covid 19 An Update What Emergency Clinicians Need To Know




Il 12 Il 23 Neutralization Is Ineffective For Alopecia Areata In Mice And Humans Journal Of Allergy And Clinical Immunology




Covid 19 Outcomes Among Racial And Ethnic Minority Individuals With Inflammatory Bowel Disease In The United States Clinical Gastroenterology And Hepatology
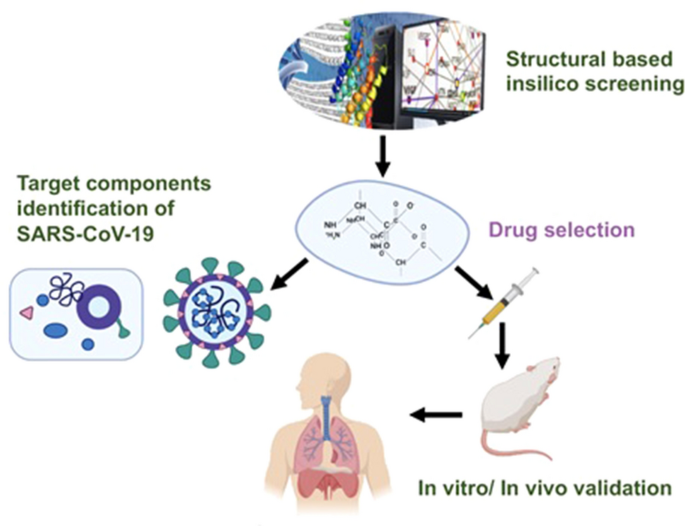



Current Strategies Against Covid 19 Chinese Medicine Full Text
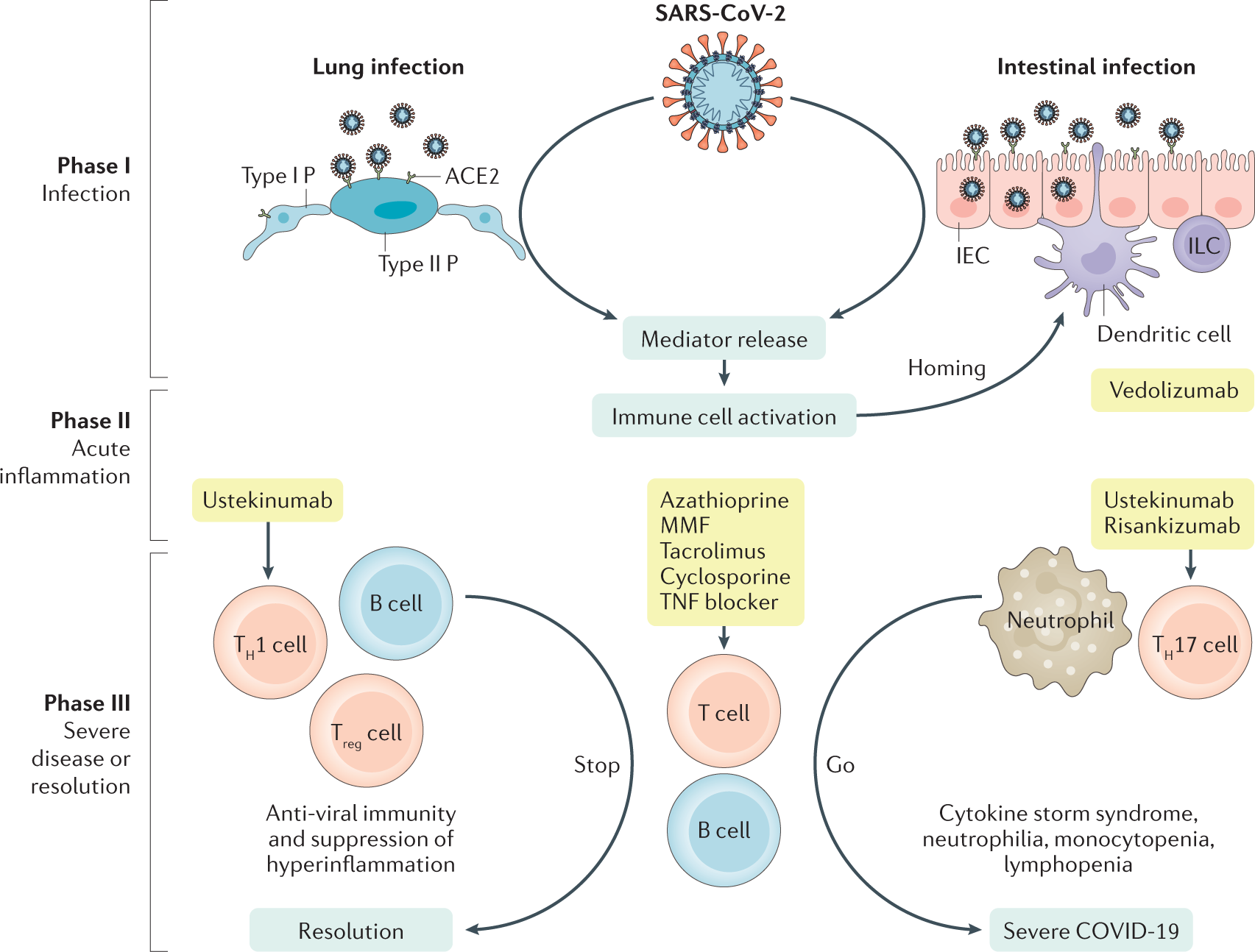



Covid 19 Biologic And Immunosuppressive Therapy In Gastroenterology And Hepatology Nature Reviews Gastroenterology Hepatology
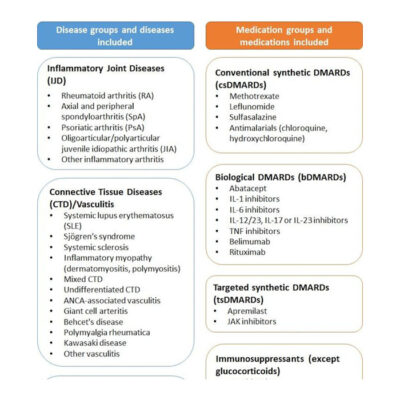



Medikamentensicherheit Drfz
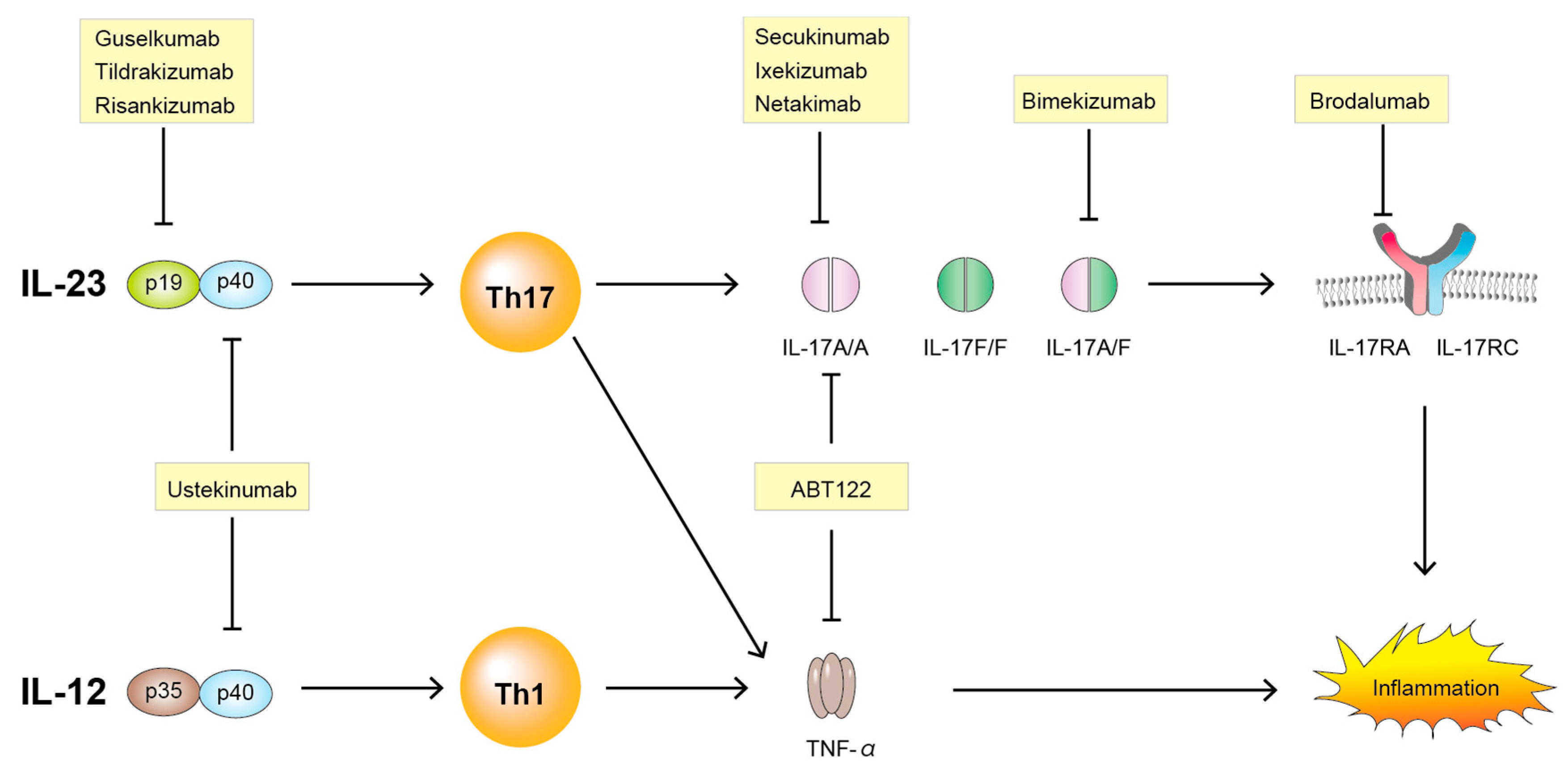



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html




Rational Combination Therapy To Overcome The Plateau Of Drug Efficacy In Inflammatory Bowel Disease Gastroenterology




Interleukin 12 And Interleukin 23 Blockade In Leukocyte Adhesion Deficiency Type 1 Nejm



Polymyalgia Rheumatica Und Riesenzellarteriitis Kortison Il 6 Therapie Was Kommt Jetzt Medmedia
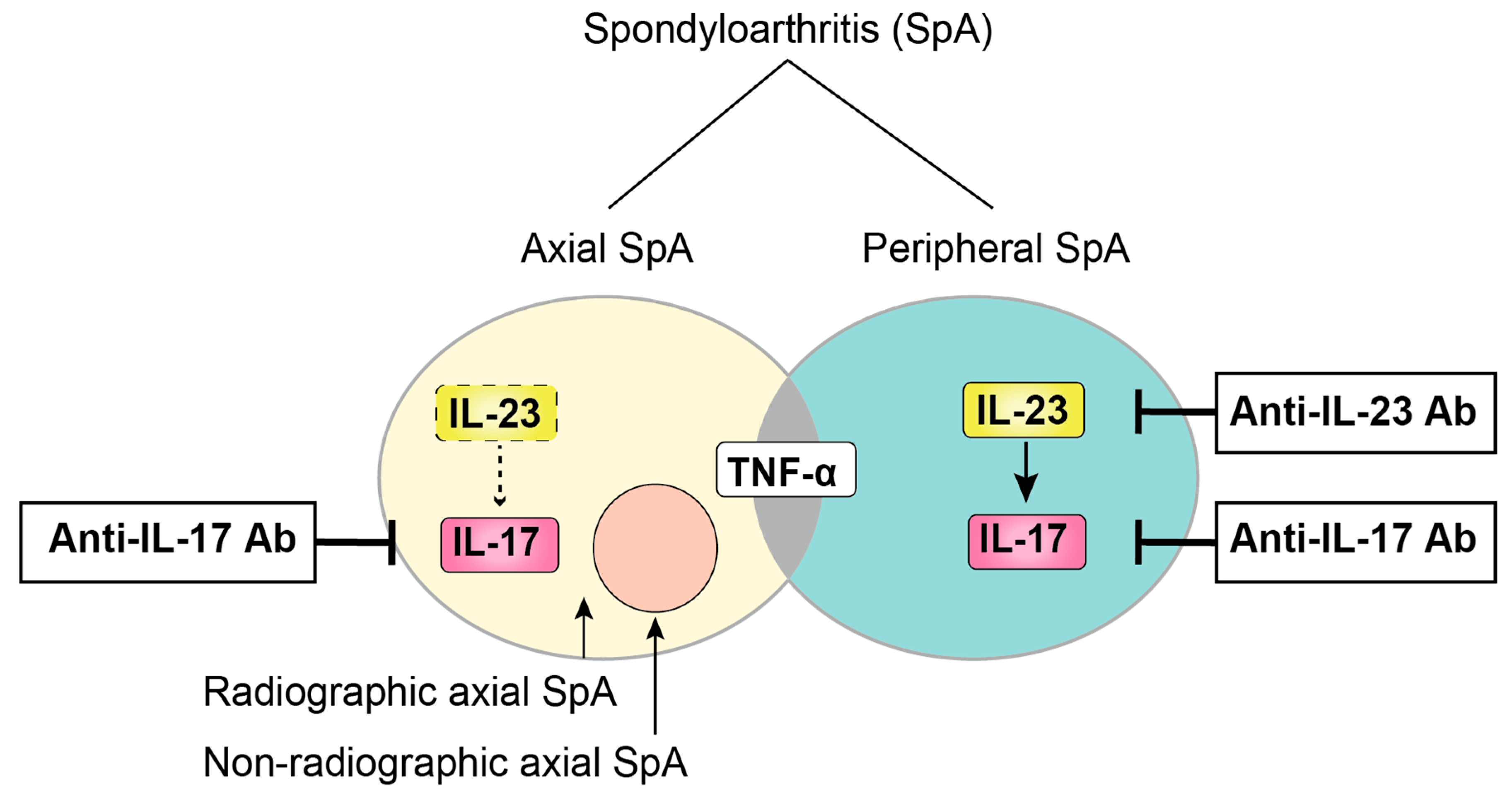



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html
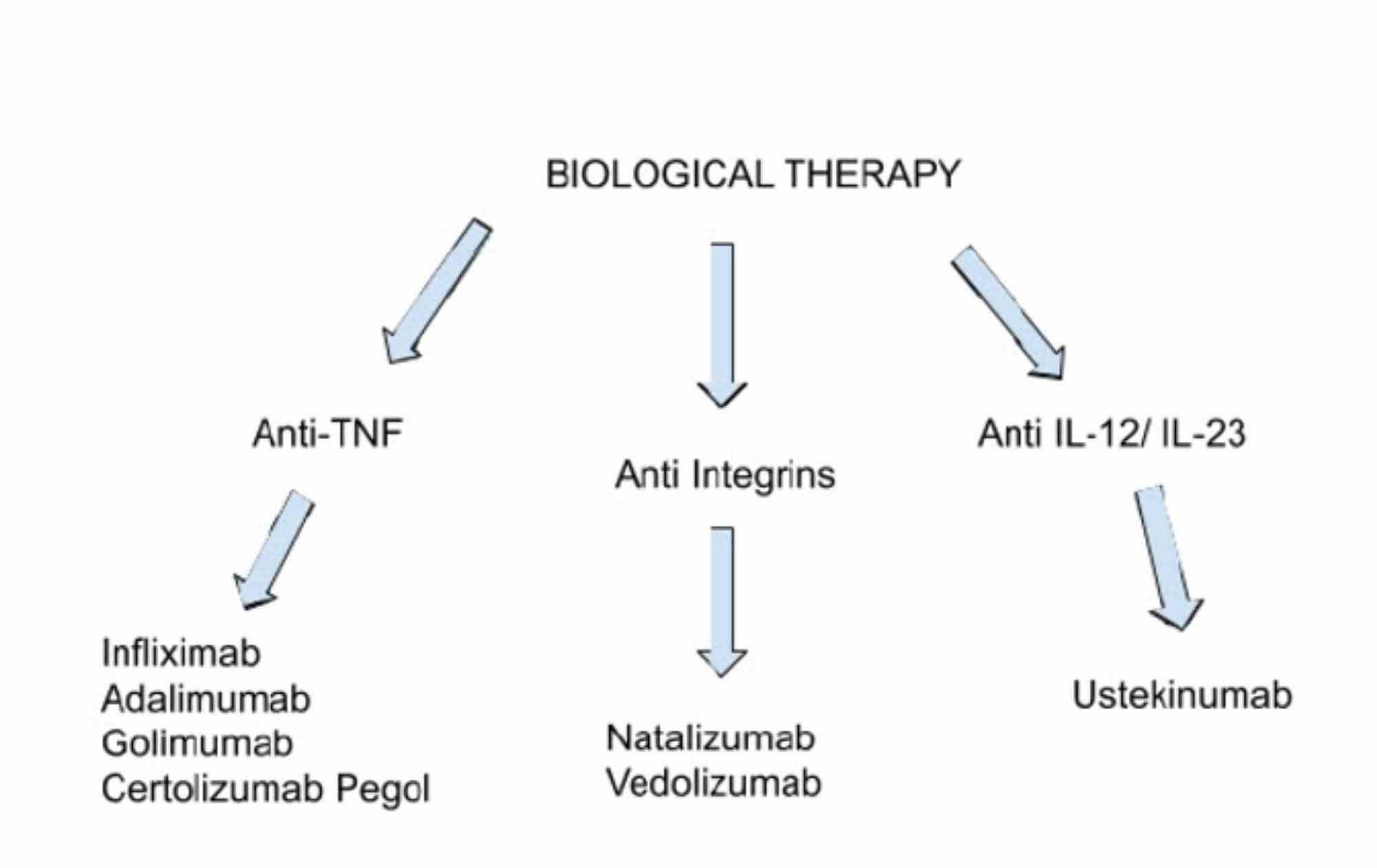



Cureus Biologics Targeting In The Treatment Of Inflammatory Bowel Disease A Conundrum




Considerations For Safety In The Use Of Systemic Medications For Psoriasis And Atopic Dermatitis During The Covid 19 Pandemic Ricardo Dermatologic Therapy Wiley Online Library




Neutralization Of The Interleukin 12 Interleukin 23 Pathways Associated Download Scientific Diagram




Could Metabolomics Drive The Fate Of Covid 19 Pandemic A Narrative Review On Lights And Shadows




Current Summary Data Secure Ibd Database
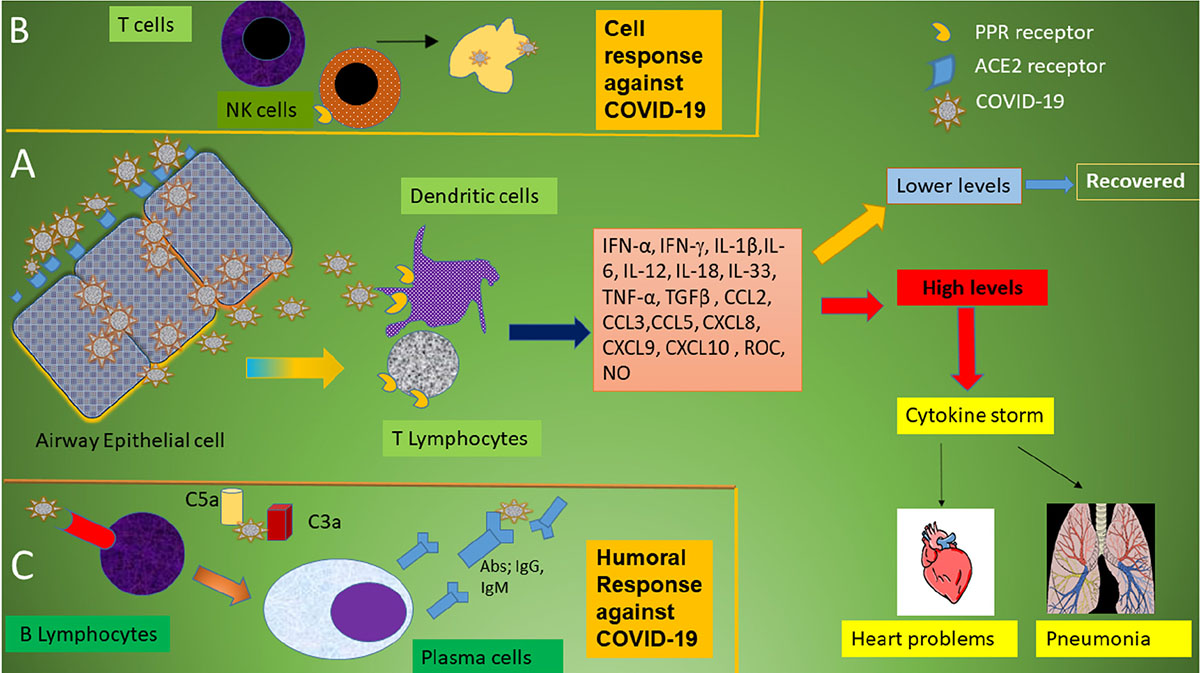



Frontiers The Immune Response And Immunopathology Of Covid 19 Immunology




The Risk Of Respiratory Tract Infections And Interstitial Lung Disease With Interleukin 12 23 And Interleukin 23 Antagonists In Patients With Autoimmune Diseases A Systematic Review And Meta Analysis Journal Of The American



2



Most Inflammatory Disease Patients On Immunosuppressants Mount A Response To The Covid 19 Vaccine




Factors Associated With Covid 19 Related Death In People With Rheumatic Diseases Results From The Covid 19 Global Rheumatology Alliance Physician Reported Registry Annals Of The Rheumatic Diseases




A Simple Home Therapy Algorithm To Prevent Hospitalisation For Covid 19 Patients A Retrospective Observational Matched Cohort Study Eclinicalmedicine
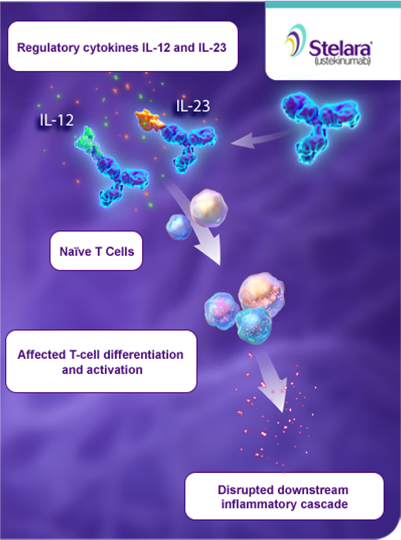



Stelara Ustekinumab Mechanism Of Action Plaque Psoriasis




Ustekinumab A Human Interleukin 12 23 Monoclonal Antibody For Psoriatic Arthritis Randomised Double Blind Placebo Controlled Crossover Trial The Lancet




Points To Consider For The Treatment Of Immune Mediated Inflammatory Diseases With Janus Kinase Inhibitors A Consensus Statement Annals Of The Rheumatic Diseases



Empfehlungen Zum Management Der Psoriasisarthritis Mit Pharmakologischen Therapien Update Der Eular Guidelines Medmedia




Interleukin 12 Il 12 But Not Il 23 Deficiency Ameliorates Viral Encephalitis Without Affecting Viral Control Journal Of Virology




Mechanism Of Action Moa Ilumya Tildrakizumab Asmn Hcp
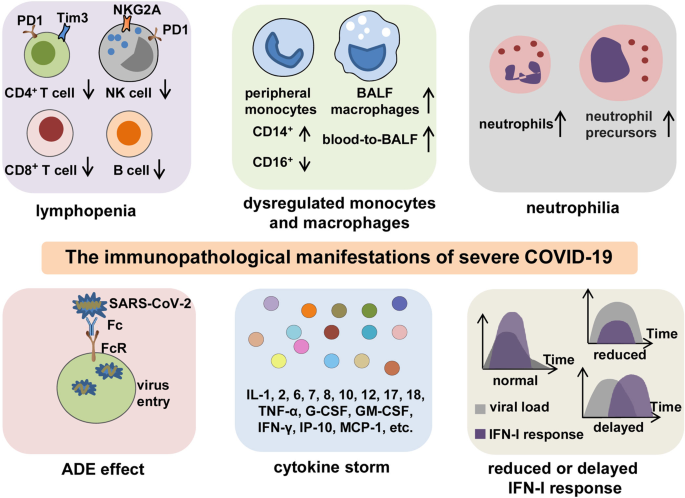



The Signal Pathways And Treatment Of Cytokine Storm In Covid 19 Signal Transduction And Targeted Therapy
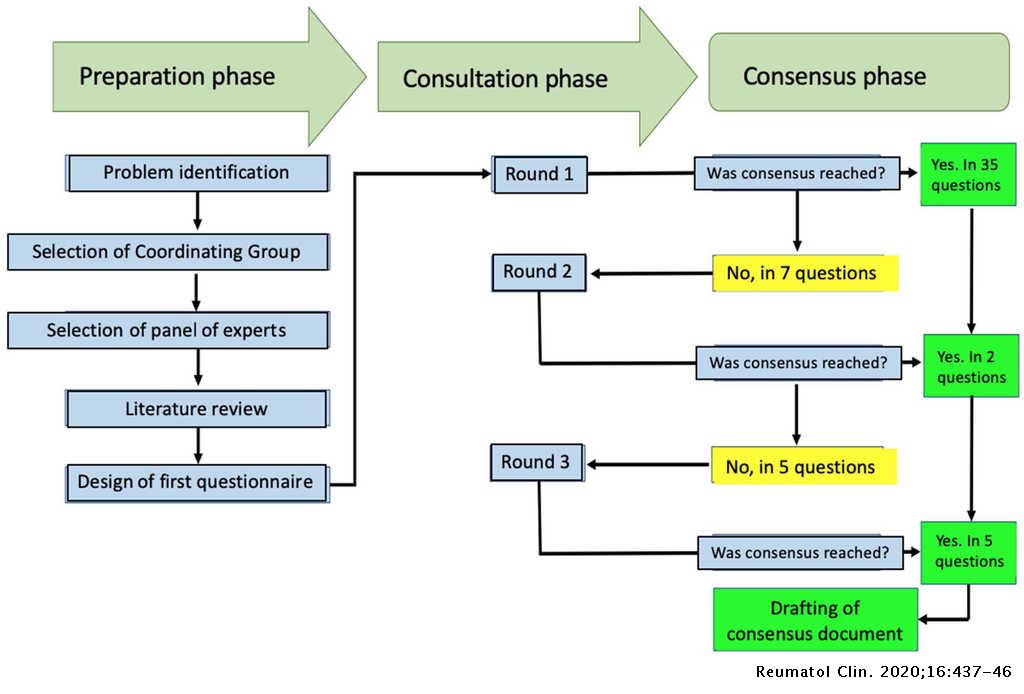



Recommendations On The Management Of Adult Patients With Rheumatic Diseases In The Context Of Sars Cov 2 Covid 19 Infection Colombian Association Of Rheumatology Reumatologia Clinica




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt



Recommendations On The Management Of Adult Patients With Rheumatic Diseases In The Context Of Sars Cov 2 Covid 19 Infection Colombian Association Of Rheumatology Reumatologia Clinica




Management Of Plaque Psoriasis A Review And Comparison Of Il 23 Inhibitors European Medical Journal




Iuis Webinar Covid 19 Immune Compromise Fais Africa
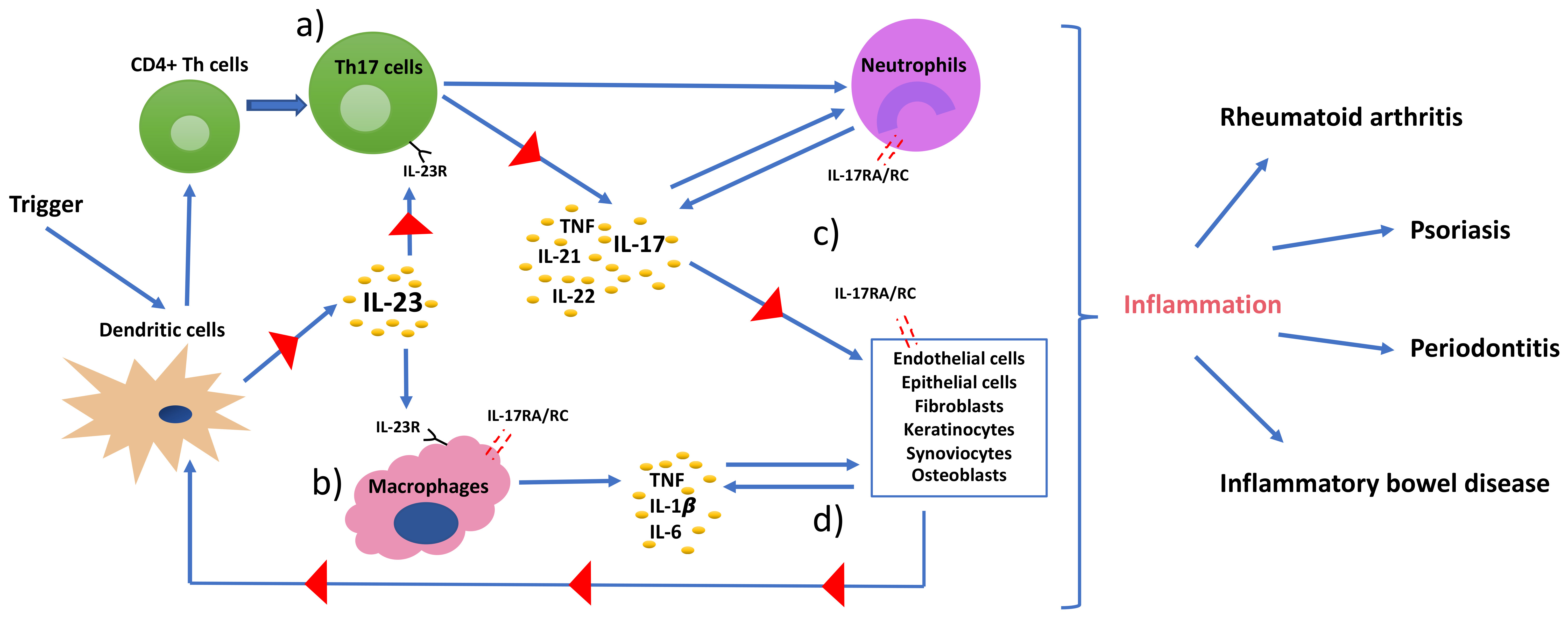



Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html




Risk Of Severe Covid 19 Outcomes Associated With Immune Mediated Inflammatory Diseases And Immune Modifying Therapies A Nationwide Cohort Study In The Opensafely Platform Medrxiv



0 件のコメント:
コメントを投稿